Securing your testing, be prepared to FDA audits and Accelerating your Time To Market.

QALmed is a flexible and scalable Application Life-cycle Management (ALM) solution tuned for health-science industry that orchestrates the complete delivery of your products, from start to finish: products/releases, requirements, specifications, tests, test campaigns, test reports and defects.
The projects are ongoing. Everybody is working hard.
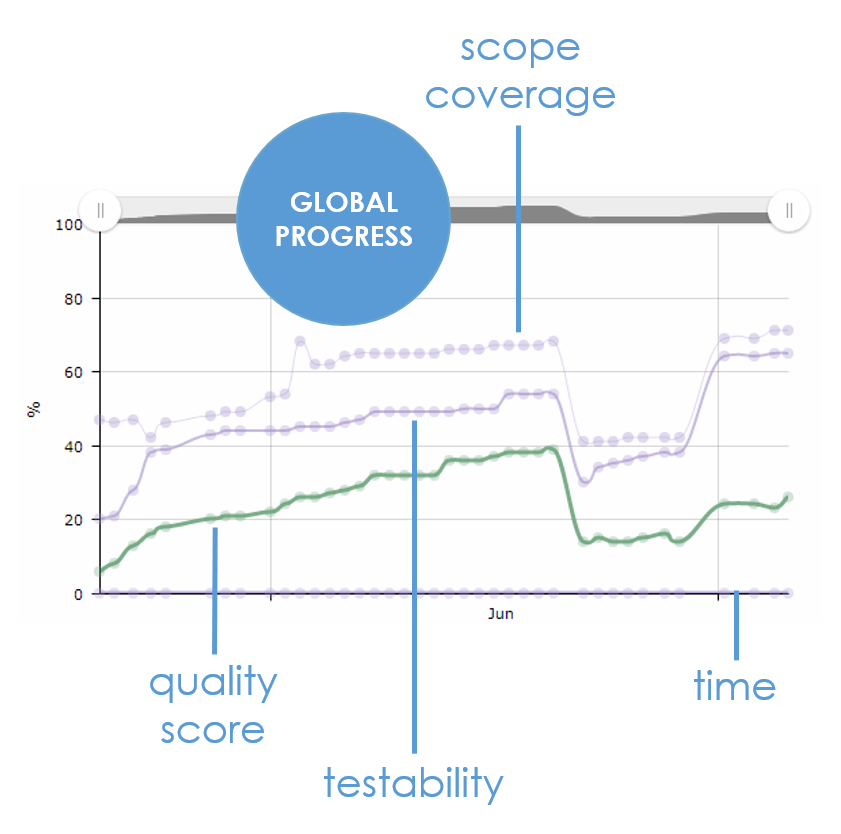
How much of the expected features and evolutions have we implemented? With which level of quality?
Are we on target? Should we drop some features?
XStudio tells you, in real-time, with its smart PROGRESS LINES chart. It covers scope, testability, quality and even specification if you manage them... Never before did you get such a clear, concise, fact-based assessment !
No excuse, you know exactly what's going on... make the right decisions now.
How much of the expected features and evolutions have we implemented? With which level of quality?
Are we on target? Should we drop some features?
XStudio tells you, in real-time, with its smart PROGRESS LINES chart. It covers scope, testability, quality and even specification if you manage them... Never before did you get such a clear, concise, fact-based assessment !
No excuse, you know exactly what's going on... make the right decisions now.
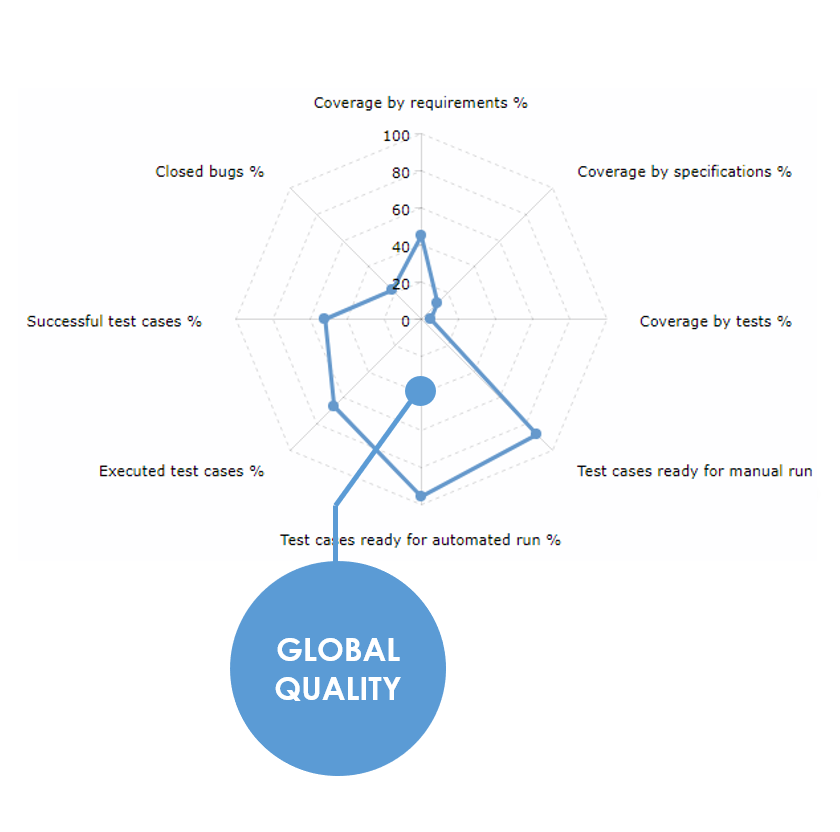
The final date is close. Someone has to make the call: are we delivering the expected value, with the right quality? Can we give it the FINAL GO?
XStudio will provide you the answer using its unique RADAR. NO gut feeling, NO guessing... just the facts !
With the RADAR, you instantly see how much scope you covered, how much you implemented and the exact quality. You even know if you are taking risks in leaving some defects.
XStudio will provide you the answer using its unique RADAR. NO gut feeling, NO guessing... just the facts !
With the RADAR, you instantly see how much scope you covered, how much you implemented and the exact quality. You even know if you are taking risks in leaving some defects.
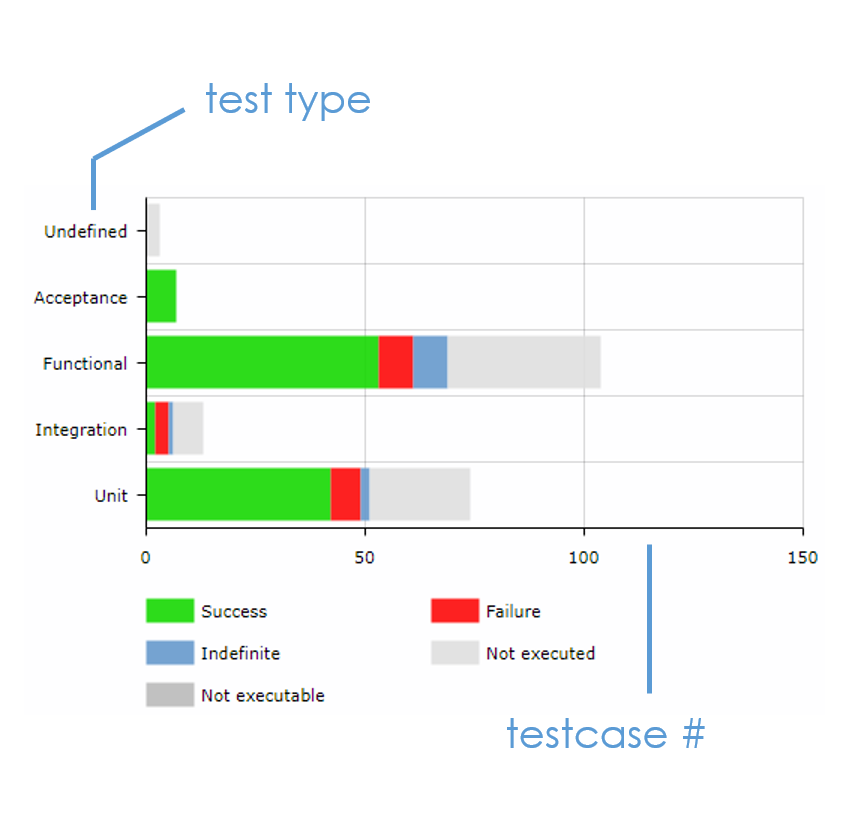
XStudio presents the TESTS DISTRIBUTION (also called tests pyramid) for each product or project.
By visualizing the spread in test types, you can immediately assess if your development cycle supports your quality objectives.
With the test TESTS DISTRIBUTION, you also see how your team is embracing proven practices such as : Test-Driven Development (TDD), Behavorial-Driven Development (BDD), Separating unit test from integration tests...
By visualizing the spread in test types, you can immediately assess if your development cycle supports your quality objectives.
With the test TESTS DISTRIBUTION, you also see how your team is embracing proven practices such as : Test-Driven Development (TDD), Behavorial-Driven Development (BDD), Separating unit test from integration tests...
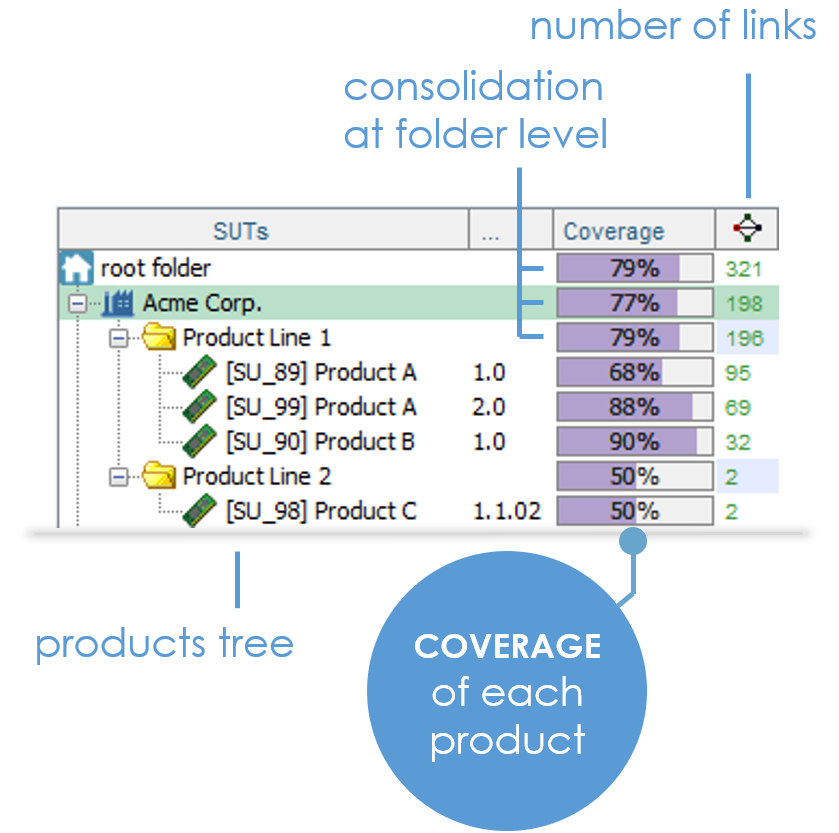
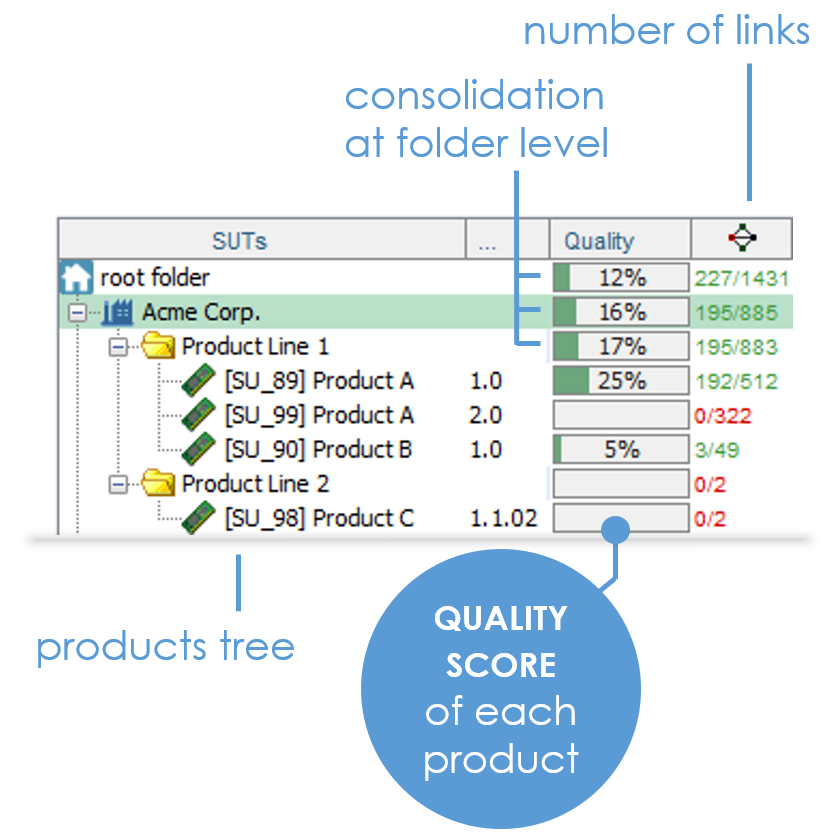
This is the first (tricky) question you'll have to answer. XStudio estimates it for you.
It takes into account all tests linked to each requirement and also allows testers to refine this coverage estimation on each individual element of the traceability matrix. We also use each item's priority to compute an as-reliable-as-possible consolidated coverage.
STOP hoping for the best ! Just make it happen.
It takes into account all tests linked to each requirement and also allows testers to refine this coverage estimation on each individual element of the traceability matrix. We also use each item's priority to compute an as-reliable-as-possible consolidated coverage.
STOP hoping for the best ! Just make it happen.
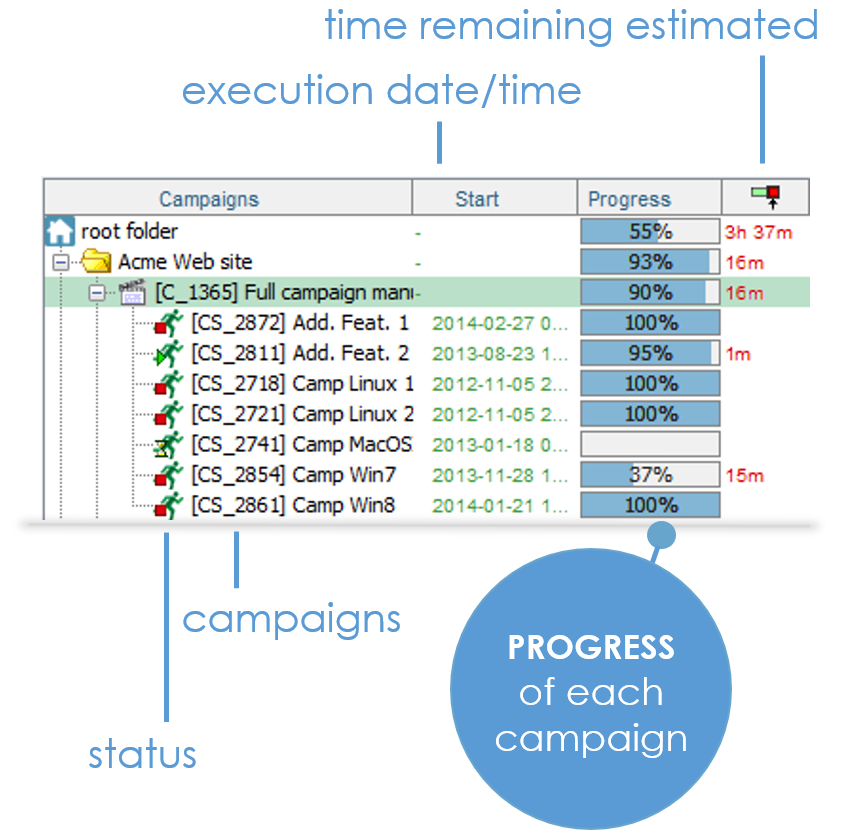
Whenever you execute manual or automated tests, XStudio records the run time, for each test.
For each new campaign, XStudio can then predict how long it should take to complete it. It will even determine pretty precisely how many days, hours and minutes remain to finish an on-going campaign.
STOP waiting for miracles ! Just plan for it.
For each new campaign, XStudio can then predict how long it should take to complete it. It will even determine pretty precisely how many days, hours and minutes remain to finish an on-going campaign.
STOP waiting for miracles ! Just plan for it.
Well, is it self-explanatory enough?
Full traceability in the whole process including design, engineering specification, development, testing and bug-fixing
All test-types are natively supported
Taking any decision should be based on the quality of the product being delivered
You should never be limited with your choices in terms of automation
Freeze and Digitial signature
Ability to Freeze and digitally signed an asset (requirment, test, result) is a stonrg requirement dictated by several certification bodies including FDA, xxx, yyy
QAlmed allow you to smoothly setup approval process and let your manager seal the data whenever it's required.
Our Signature Control Center module even facilitate more auditor's job.
Be prepared for audits andhave your proofs ready!
QAlmed allow you to smoothly setup approval process and let your manager seal the data whenever it's required.
Our Signature Control Center module even facilitate more auditor's job.
Be prepared for audits andhave your proofs ready!


Detailed Audit Log
When testing Medical device, Life Science and Pharma product you must be prepared to trace any action as well as who did it and when. This is an essential aspect of traceability.
Maitrise des Approvals et des droits
blah blah blash.....


Versioned Documents & Reports Management
Including timestamped and digitally signed reports.

An ALM entirely designed for Medical, Life Science and Pharma
We don't play with health. QALmed simplifies your preparation for regulatory audits:
- FDA Title 21 CFR
- EU MDR
- EU GMP Annex 11
- DO-178C (DAL A/B/C)
- ISO 26262 (ASIL A/B/C/D)
- IEC 61508 (SIL 1/2/3/4)
- EN 50128 (SIL 0/1/2/3/4)
- IEC 60880
- ISO 9001
- ISO 27001
It also helps you to conform to following standards:
- IEEE 830
- IEEE 829
- IEEE 1028
- IEEE 1044
- ISO 25000 (SQuaRE)
- GDPR, RGPD
It also enables your practices with methodology standards such as:
- CMMI
- TMMi